dry heat autoclave spore test|biological autoclave testing : bulk por units, or Bacillus atrophaeus for dry heat autoclaves). Inac-tivation of the BI strongly implies that other potential pathogens in the load have been killed. In other words, if Geobacillus and . A passing spore test is called a negative spore test. If both the test and control BI are killed, this is an indication that . In autoclave, the method of killing the microbes is by the usage of moist heat and pressure. The Autoclave design and principle is referred from pressure cooker. And hence, in general, the autoclave is also called as .First, the one that can be placed in your workplace is a tabletop autoclave or bench autoclave. On the other side, the Autoclave that is not portable and cannot be placed on the floor is known as the floor autoclave.
{plog:ftitle_list}
Infectious waste must be treated in an autoclave for a minimum of 30 minutes at 121°C (250°F); however, the total processing time required to decontaminate infectious waste .Page 25 Printout is possible in four modes. The mode shown below is preset before delivery and is recommended by GETINGE. The system stores the last process so that an “emergency printout” can be produced afterwards if there .
This monitoring process assesses the killing of highly resistant microorganisms (e.g., Geobacillus stearothermophilus for autoclaves and chemical vapor units, or Bacillus atrophaeus for dry heat autoclaves). Positive spore test results are a relatively rare event 838 and can be attributed to operator error, inadequate steam delivery, 839 or equipment malfunction. Portable (table-top) steam sterilizers are used in outpatient, dental, and rural clinics. 840 These sterilizers are designed for small instruments, such as hypodermic syringes and needles .por units, or Bacillus atrophaeus for dry heat autoclaves). Inac-tivation of the BI strongly implies that other potential pathogens in the load have been killed. In other words, if Geobacillus and . A passing spore test is called a negative spore test. If both the test and control BI are killed, this is an indication that .In ethylene oxide and dry heat sterilization, the test organism used is typically the endospore-forming bacteria, Bacillus atrophaeus, (ISO11138-2 Sterilization of health care products – Biological indicators . To verify the spore population of the test organism, samples of biological indicators are selected from each distinct manufacturer .
Mail in your spore test and get results within 24 hours (Steam), 72 hours (Chemical Vapor), 7-days (Dry Heat or EtO Gas). Product code: BI-SA26-Kit These tests monitor sterilization processes in Steam Autoclaves and Ethylene Oxide (EtO) Sterilizers.and Dry . Biological indicators for hydrogen peroxide sterilization monitoring include self-contained biological indicators, test packs or challenge packs. AAMI ST58:2013 recommends using a PCD with a BI daily, preferably every cycle, for monitoring sterilizers and in every load containing implants. Types of Biological Indicators for Sterilization Processes
There are three ways to spore test your autoclave. Mail it out weekly/monthly to a spore test lab and get results (usually takes 1-2 weeks) . EtO Gas and Dry Heat Sterilizers. Results within 48 hours after the indicator arrives in the lab. 7 /kit of 12 tests. Q: What are the next steps after a failed autoclave spore test? A: After a failed result, it’s crucial to make sure the autoclave is working properly. If there are no impediments to operation — such as a clogged strainer drain or a blocked drain port — adjust sterilization parameters (e.g. time, temperature, etc.) and run the cycle again . It is important to spore test autoclaves to assess whether or not autoclaves are functioning properly. Spore tests contain nonpathogenic bacterial spores that are highly resistant to heat. . Heat sterilizers either use dry heat or steam with maximum temperatures above 250 degrees Fahrenheit (121 degrees Celsius) to kill microorganisms . It is crucial to spore test an autoclave to make sure that the autoclave is sterilizing equipment properly. Spore tests, . Heat sterilizers either use dry heat or steam with maximum temperatures above 250 degrees Fahrenheit (121 degrees Celsius) to kill microorganisms. Autoclaves use the steam heat method and are the most widely-used .
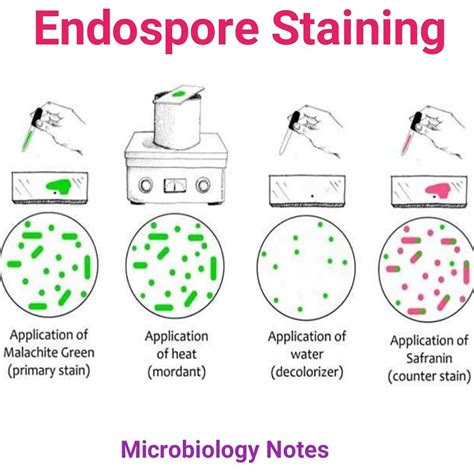
The most commonly used forms of BIs in dentistry are spore strips, and self-contained spore vials. Self-contained vials have a glass ampule of sterile media, which is encased in a plastic vial with a spore strip inside. After sterilization, the vial is crushed—allowing the media to come into contact with the spore strip and then incubated.
spore testing procedure
Dual species spore strips with matching control. Works with any commercial sterilizer. Results reported via email in 1 day for steam, 3 days for chemical, and 7 days for dry heat and EtO sterilizers. Immediate phone and email notification of failed tests.In December 2003, the CDC published a major consolidation and update of its infection control recommendations for dentistry. 2 The 2003 document incorporated relevant recommendations that were previously published in several other CDC documents and contained an extensive review of the science related to dental infection control. In March 2016, the CDC issued the .At 170°C, even the most resistant microbial spore form will have a D value of 6 to 10 min. . ENDOTOXIN CHALLENGE IN THE VALIDATION OF DRY-HEAT STERILIZERS . Heat penetration test containers of corresponding container size and type and fill volume shall be placed adjacent to the challenge test items in each run.Dry Heat Sterilizer Spore Monitoring Mail-In Test Kit SKU: 400633 SKU: 400633 (0) Regular price 2.00 . In office spore monitoring mail in test kit for dry heat sterilizers. Includes 12 Tests. For use with steam, chemical vapor, dry heat or EO gas sterilization processes.
Single spore test. Single Test. SporView Culture Set. Green Card. Spore test 12pk. Weekly Test. Record books to monitor spore test. Steri Pack LF. . COX dry heat sterilizer, Pelton and Crane OCM, OCR; Distributors of Sterilizers & Autoclaves.
Spordi Biological Indicator Strips are specifically designed for determining the effectiveness of steam, ethylene oxide (EO), or dry heat. Each strip is made from special filter paper inoculated with both Bacillus atrophaeus (BA) and Geobacillus stearothermophilus (GST) bacterial spores.
Q - How do you make sure that the instruments in your office are sterile? A - Do a spore test on a regular basis.. Important information from The Centers for Disease Control (CDC) vol. 42/No. RR-8 . Proper functioning of sterilization cycles should be verified by the periodic use (at least weekly) of biological indicators (i.e. spore tests).
spore testing incubation temperature
spore test for sterilization
negative spore test autoclave
Dry heat sterilizers come with a special ventilation system. The air in the chamber is heated evenly thereby preserving sterilized objects. The temperature inside the sterilizer is supported and the possibility of overheating is eliminated. For Dry Heat Sterilizers, use Bacillus Atrophaeus spore test strips. If you have any questions about the SteriDent Static Heat Sterilizer Model #200, any other CPAC units or autoclaves in general, please give us a call at 704-966-1650 option 3 .
ConFirm™ Value Test Service Mail-in Sterilizer Indicators Monitoring Service- Compatible with steam, EtO, chemical vapor and dry heat sterilizers. Spore strips contain highly resistant dual-species bacterial spores providing the most reliable method of .
Mesa Labs makes it easy to test and track results with our anytime compliance dashboard. The dashboard gives you oversight across multiple offices or facilities, allows easy result sorting and reporting over time, and simplifies your compliance program from start to finish. . and chemical or dry heat indicators. Autoclave Mail-In Spore .
ConFirm® is an in-office system for monitoring steam sterilizers . Simply process test indicator vial in a normal steam sterilization cycle, remove, crush ampoule and place in incubator along with an unprocessed control vial for required 24 hour incubation period . SporView® Sterilization Biological Indicator Kit Steam / EO Gas / Dry Heat .Spore test strips also provide an effective way to biologically monitor both mechanical function and operator technique for steam and dry heat sterilizers; The Centers for Disease Control (CDC), the Association for the Advancement of Medical Instrumentation (AAMI), the American Dental Association (ADA) and the Organization for Safety and .Order McKesson Sterilizer Monitoring Mail-In Service Steam / EO Gas / Dry Heat / Chemical Vapor by McKesson Brand 73-PP012 Pharmaceuticals . McKesson Sterilizer Monitoring Mail-In Service Steam / EO Gas / Dry Heat / Chemical Vapor TEST SYSTEM, SPORE MAIL-IN (12/BX 10BX/CS) Previous. Next . Previous. Next . Product Images .
For dry heat sterilization, many use the term F H where the reference temperature is 170 °C and the z-value is 20 °C. Some companies use the term F P for dry heat that is used also to depyrogenate, using a reference temperature of 250 °C and a z-value between 40 and 50 °C (Agalloco 2008). Dry heat sterilizers only require electricity to operate and depending on the manufacturer, some units run on 110V and some require 220V. . (Quality Systems Regulations) guidelines. Lab technicians who are not careful can contaminate the spore test during transfer and culturing, especially with Bacillus subtilis spores, which are incubated at .vendor. Make sure you are purchasing biological spore indicators and not just heat test strips. You can search “biological indicator” on the internet and find results. OPTIONS: o In Office: If perform your testing in office, you will purchase the ampules from a vendor AND have to process the test ampule, incubate, read and record the results
is the tcole test hard
is the teacher certification test hard
Once the cycle type is determined you must decide on the key cycle parameters: sterilization temperature and sterilizer time. The vast majority of loads sterilized in the laboratory setting .
dry heat autoclave spore test|biological autoclave testing